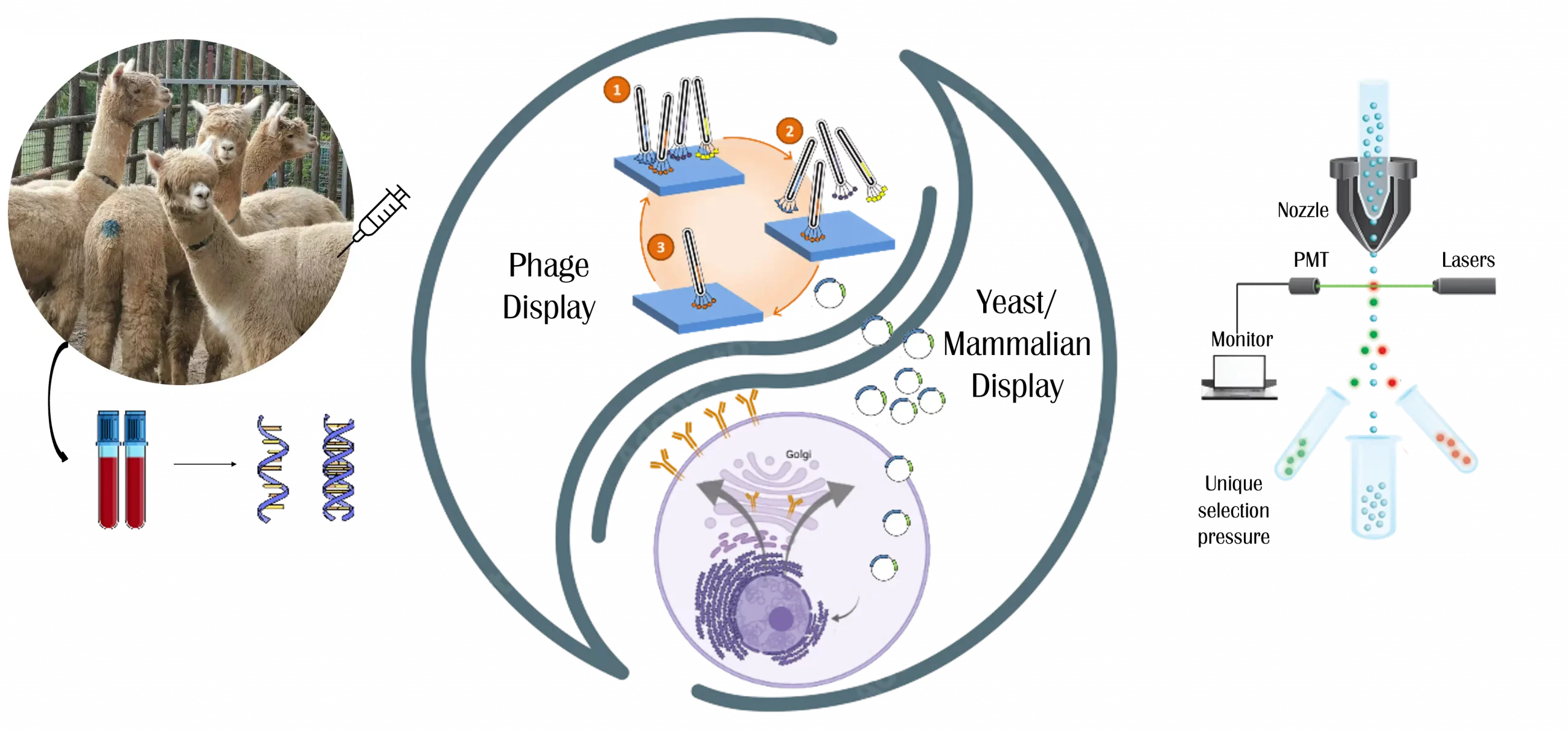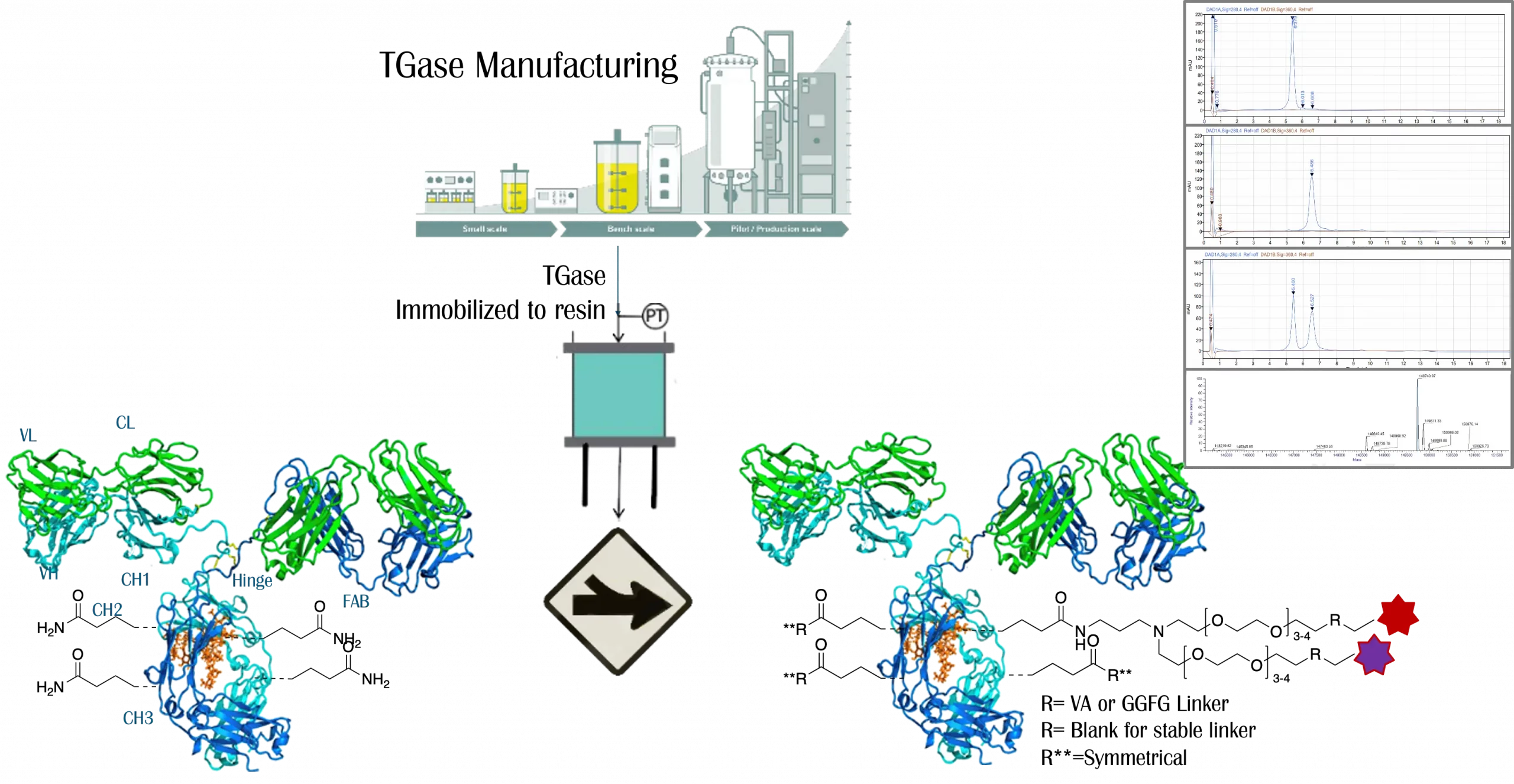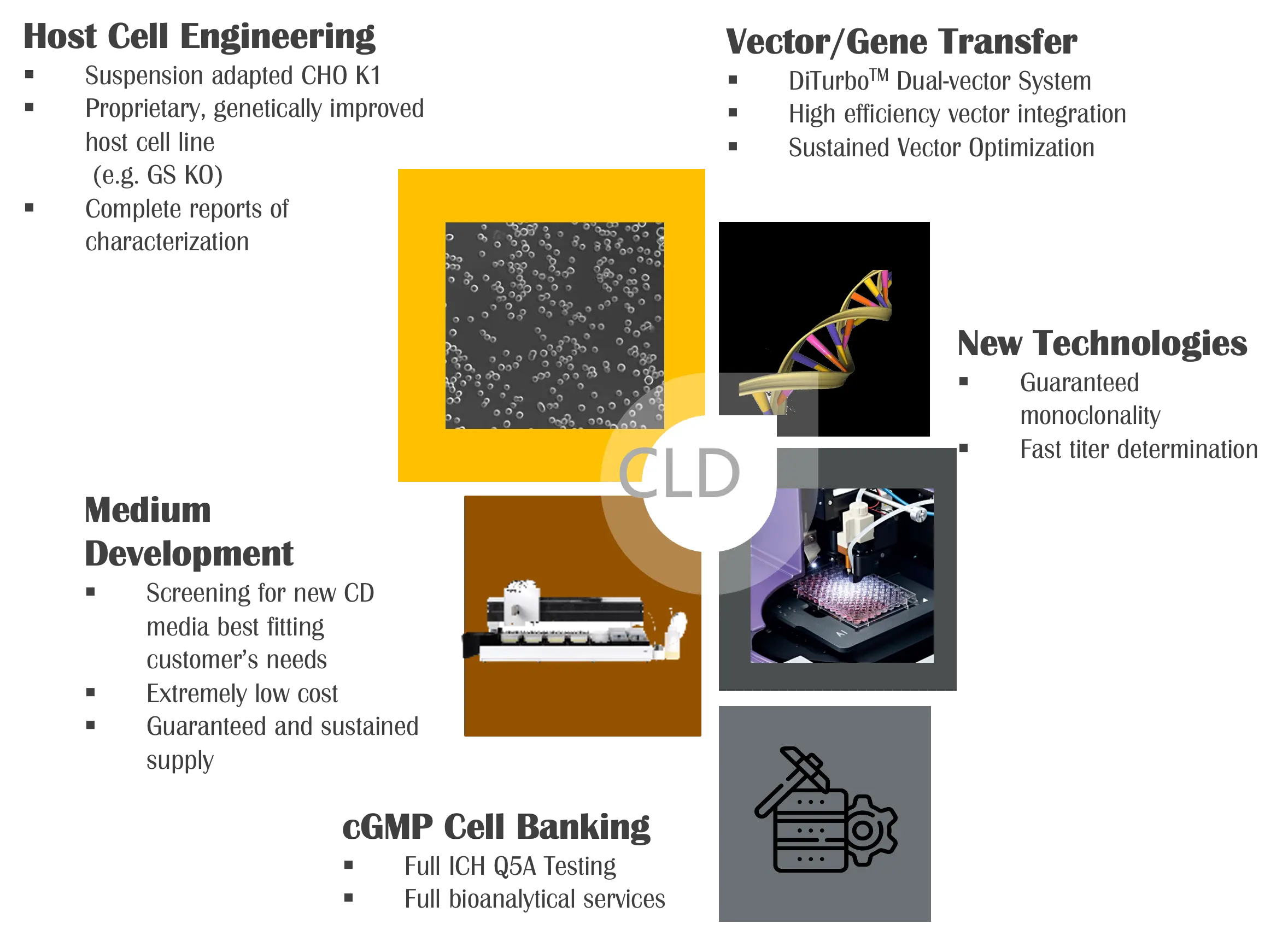
Legato Biologics offers comprehensive Cell Line Development (CLD) services, leveraging robust host cell lines such as CHO K1 and CHO K1 GS-KO to support diverse biopharmaceutical applications. Utilizing the proprietary DiTurbo™ dual-vector transfection system, Legato Biologics executes efficient gene expression and accelerated cell line establishment, ensuring high productivity and stability. The company’s streamlined development workflow, achieving PCB (Production Cell Bank) generation in 3 to 4 months from DNA sequence, reflects its commitment to speed and efficiency in bioprocess development.
With a proven track record of 20+ successful cell line development projects, Legato Biologics consistently achieves high product titers, up to 10 g/L, making it a preferred partner for both early-stage and late-stage biologics development. The company’s high-throughput platform facilitates the selection of optimal cell lines tailored for fed-batch processes with robust performance and scalability. Our data-driven approach enables precise cell line selection to meet specific production needs and regulatory requirements.
Legato Biologics’ CLD capabilities emphasize rapid and reliable recombinant cell line development with a strong focus on clonality assurance. The integration of a single-cell imager and high-throughput detector allows thorough characterization and documentation of clonality, fulfilling regulatory expectations and accelerating the transition to clinical and commercial manufacturing. These advanced tools empower Legato Biologics to deliver high-quality, scalable solutions that support the evolving demands of the biopharmaceutical industry.
Legato Biologics’s CLD capabilities:
- Robust host cell lines, including CHO K1 and CHO K1 GS-KO, optimized for high-yield and stable protein expression.
- DiTurbo™ dual-vector transfection system, enabling efficient gene delivery and rapid cell line establishment with enhanced productivity.
- Accelerated development timeline, achieving production cell bank (PCB) generation in 3 to 4 months from DNA sequence.
- Proven expertise with over 20 successful cell line development projects, achieving product titers of up to 10 g/L.
- High-throughput screening platform for precise selection of cell lines optimized for fed-batch production processes.
- Rapid and reliable recombinant cell line development with a clonality guarantee, supported by state-of-the-art single-cell imaging and high-throughput detection technologies.