Full CMC Service for Monoclonal Antibody, Bispecific Antibody, and ADC Development from IND to Phase 2 Trials
Proprietary & Industry-leading uni-Qon™ Site-specific ADC Mono- & Dual-payload Conjugation
Gene Synthesis to eCTD Filing Within 11 Months for Bispecific Antibody IND Submission with FDA
From our CEO

Legato Biologics:
An Arising and Seamless CRDMO Solution
Legato Biologics embodies the essence of “smooth and connected performance,” offering an uninterrupted and fully integrated CRDMO solution to accelerate biologics development from discovery to commercialization. Our integrated platforms ensure a streamlined workflow, eliminating common bottlenecks and inefficiencies. With Legato Biologics, clients would experience a cohesive journey through every stage of development, from early research and process optimization to clinical manufacturing and regulatory support.
At Legato Biologics, we provide a comprehensive suite of services tailored to meet the evolving needs of biopharmaceutical innovators. Our cutting-edge capabilities in cell line development, process optimization, analytical testing, and GMP-compliant manufacturing ensure consistency and reliability at every phase. By integrating state-of-the-art technologies with extensive industry expertise, we help our partners achieve faster turnaround time and higher quality outcomes, all within a cost-effective framework.
What sets Legato Biologics apart is our unwavering commitment to quality and operational excellence. Our expert teams work collaboratively across disciplines to ensure seamless transitions between development milestones, reducing risks and enhancing project efficiency. Whether you’re developing monoclonal antibodies, bispecifics, or more complex modalities such as ADCs and nanobodies, Legato Biologics provides the infrastructure and expertise to bring your vision to life.
With Legato Biologics, you may expect a smooth, uninterrupted path to success—where innovation, speed, and quality converge to power your journey from concept to clinical reality with confidence.
Technologies

Legato Biologics’s CLD capabilities
Legato Biologics’s upstream process development capabilities are built upon state-of-the-art infrastructure and an experienced team dedicated to delivering high-quality biopharmaceutical products.
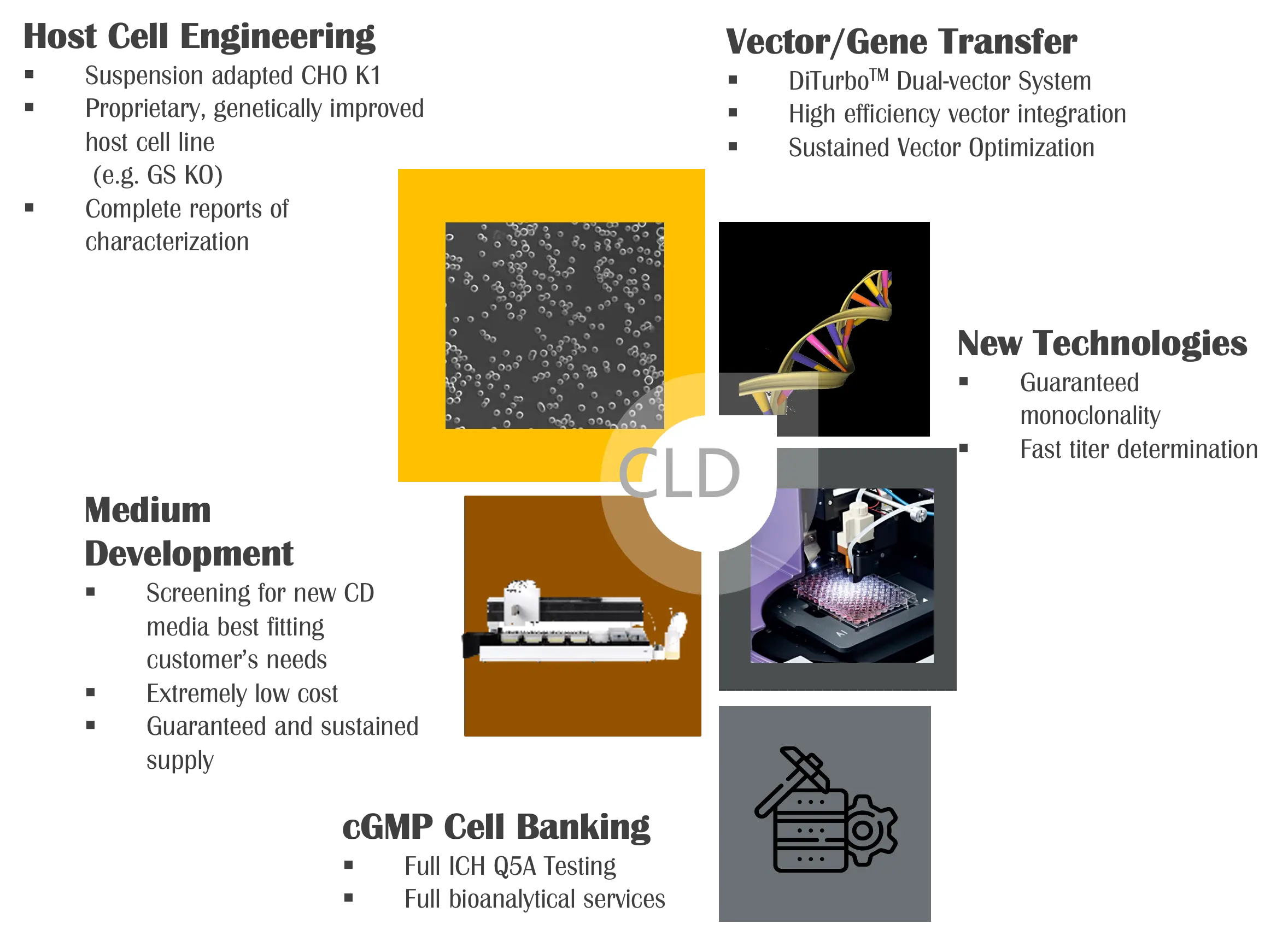
DiTurbo™ Empowered Stable Cell Line Development
Legato Biologics offers comprehensive Cell Line Development (CLD) services, leveraging robust host cell lines such as CHO K1 and CHO K1 GS-KO to support diverse biopharmaceutical applications. Utilizing the proprietary DiTurbo™ dual-vector transfection system, Legato Biologics executes efficient gene expression and accelerated cell line establishment, ensuring high productivity and stability.
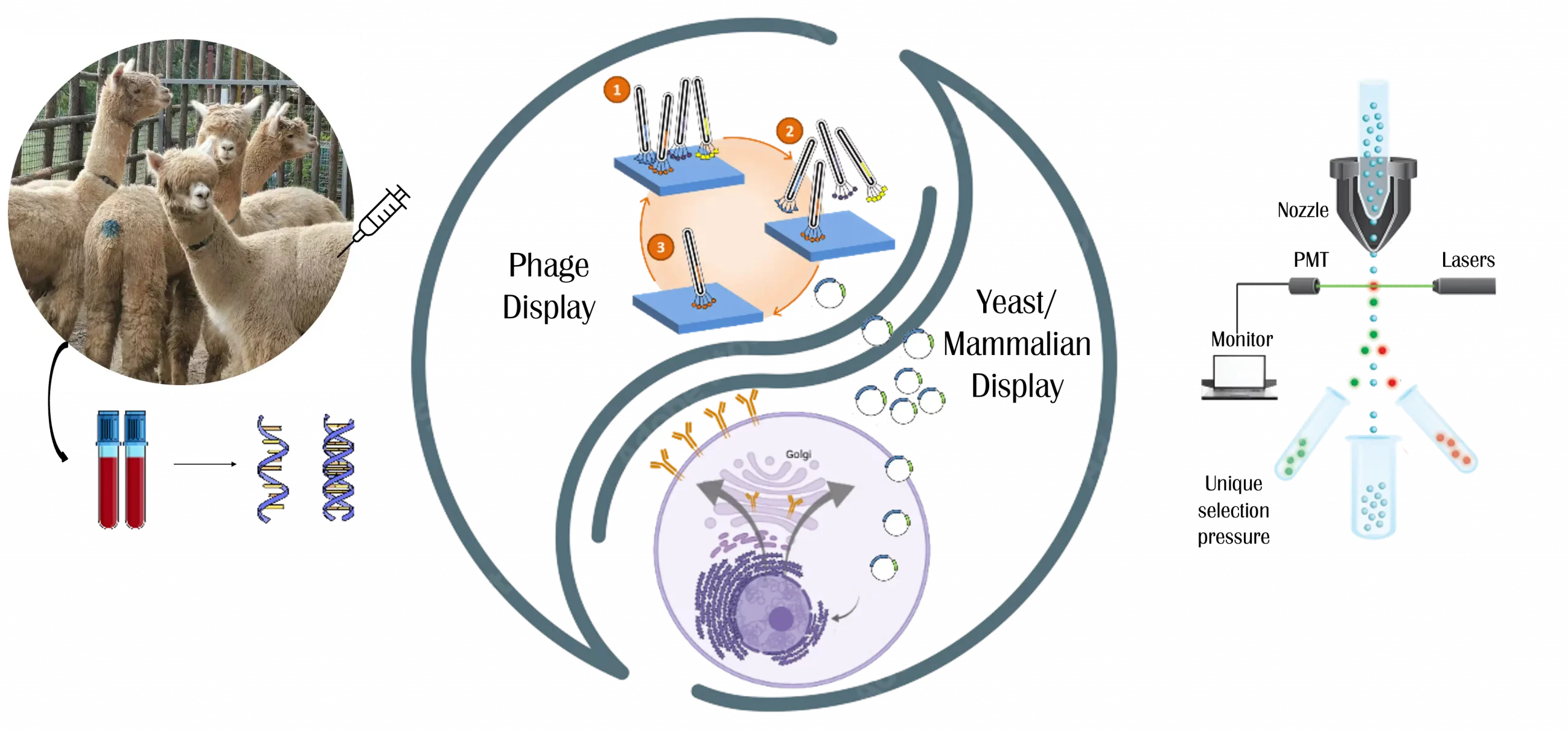
Legato Biologics’s SparxNano™ Technology: Accelerating Nanobody Discovery
SparxNano™ technology is a cutting-edge platform designed to rapidly identify high-affinity, highly selective nanobodies targeting specified extracellular proteins within just four months.